Lung and bronchus cancer is the second most prevalent form of cancer in the United States.1 Representing 12.7% of all new cancer cases, lung cancer was diagnosed in 228,820 individuals in 2020.1 It is the leading cause of cancer-related mortality in men and women, accounting for 22.4% of all cancer deaths.1
Non–small-cell lung cancer (NSCLC) accounts for 80% to 85% of all cases of lung cancer in the United States.2 The 5-year relative survival rate for patients with metastatic NSCLC is only 7%, based on data from 2010 to 2016.3 Moreover, patients with NSCLC commonly experience dyspnea, cough, fatigue, distress, and pain—symptoms that can have a substantial impact on daily functionality and quality of life.4
The treatment of metastatic NSCLC generally includes chemotherapy, radiation therapy, chemoradiation, immunotherapy, targeted therapy, or a combination of these therapies.5 Approximately 3% to 4% of patients with NSCLC harbor a mutation that leads to mesenchymal-epithelial transition (MET) exon 14 skipping mutation.6-8 The prognosis is poor in these patients, and they have limited treatment options.6,9 Recently, a targeted kinase inhibitor became the first therapy to receive approval specifically for this patient population.10
FDA Approved Capmatinib for Certain Types of Metastatic NSCLC
On May 6, 2020, the US Food and Drug Administration (FDA) approved capmatinib (Tabrecta; Novartis) for the treatment of adults with metastatic NSCLC whose tumors have a mutation that leads to MET exon 14 skipping, as detected by an FDA-approved test.10 Capmatinib was granted an accelerated approval and was designated as a breakthrough therapy and an orphan drug by the FDA.10
“Lung cancer is increasingly being divided into multiple subsets of molecularly defined populations with drugs being developed to target these specific groups,” said Richard Pazdur, MD, Director, FDA’s Oncology Center of Excellence. “Tabrecta is the first approval specifically for the treatment of patients with non–small-cell lung cancer whose tumors have mutations that lead to MET exon 14 skipping. This patient population now has an option for a targeted therapy, which they didn’t have prior to today,” Dr Pazdur commented on the approval of capmatinib for this indication.10
Mechanism of Action
Capmatinib is a kinase inhibitor that targets and blocks the activity of MET, including the mutant variant produced by exon 14 skipping. In preclinical studies, capmatinib inhibited cancer cell growth driven by a mutant MET variant lacking exon 14 and showed antitumor activity in tumors with either a mutation leading to MET exon 14 skipping or MET amplification.11
Capmatinib inhibits MET phosphorylation and MET-mediated downstream signaling proteins and blocks the proliferation and survival of MET-dependent cancer cells.11
Dosing and Administration
The recommended dose of capmatinib is 400 mg taken orally twice daily with or without food. Capmatinib is available as a 150- or 200-mg tablet.11
Capmatinib can be taken with or without food; the tablets should be swallowed whole and should not be cut, crushed, or chewed.11
Pivotal Clinical Trial: GEOMETRY mono-1
The efficacy of capmatinib was evaluated in the GEOMETRY mono-1 study, a nonrandomized, open-label, multicenter, phase 2 clinical trial that included 97 patients (median age, 71 years; range, 49-90 years) with NSCLC; most of the patients were female (60%), white (75%), and had an Eastern Cooperative Oncology Group performance score of 0 (24%) or 1 (75%).6,11
Eligible patients were required to have tumors with the mutation leading to MET exon 14 skipping, as detected by local tests, and confirmed with an FDA-approved test. Of the patients who had received previous treatment, 88% had received previous platinum-based chemotherapy. Patients received capmatinib 400 mg orally twice daily until disease progression or unacceptable toxicity.11
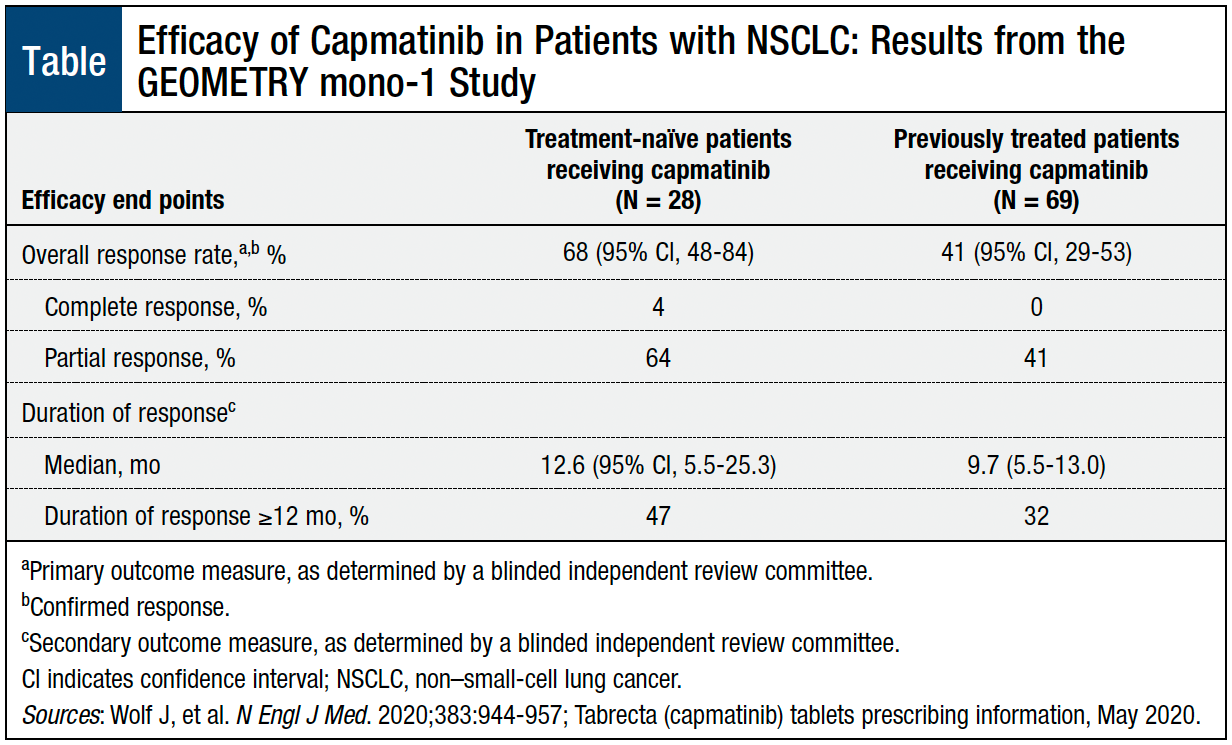
The overall response rate in patients who received capmatinib was 68% in treatment-naïve patients and 41% in previously treated patients (Table).6,11 Of patients who achieved a response, the median duration of response was 12.6 months in the treatment-naïve group and 9.7 months in the previously treated group.6,11
Adverse Reactions
The most common (≥20%) adverse reactions associated with capmatinib in patients with NSCLC with MET exon 14 skipping were peripheral edema (52%), nausea (44%), fatigue (32%), vomiting (28%), dyspnea (24%), and decreased appetite (21%).11
Serious adverse reactions were reported in 51% of patients who received capmatinib, and 16% of patients permanently discontinued treatment as a result of adverse reactions, which included peripheral edema (1.8%), pneumonitis (1.8%), and fatigue (1.5%). A fatal adverse reaction attributed to pneumonitis was reported in 1 patient treated with capmatinib.11
Drug Interactions
The coadministration of capmatinib with a strong cytochrome P450 3A (CYP3A) inhibitor increases the exposure of capmatinib, which may increase the occurrence and severity of adverse reactions. Strong CYP3A inhibitors should not be coadministered with capmatinib.11
Conversely, the coadministration of capmatinib with a strong or moderate CYP3A inducer can decrease the exposure of capmatinib, which may reduce antitumor activity. Strong or moderate CYP3A inducers should not be coadministered with capmatinib.11
Coadministration of capmatinib with CYP1A2 substrates, P-glycoprotein substrate, breast cancer resistance protein substrate, and human multidrug and toxin extrusion (MATE) 1 and MATE2-K substrates may increase the adverse reactions of these substrates.11
Capmatinib has no contraindications.11
Use in Specific Populations
No data are available on the use of capmatinib in pregnant women. Capmatinib can cause fetal harm when administered to a pregnant woman.11
Data are not available on the presence or effects of capmatinib or its metabolites on the breastfed child or on milk production. Women should not breastfeed during capmatinib treatment and for 1 week after the final dose of capmatinib.11
Capmatinib was not associated with overall differences in safety or effectiveness between patients aged ≥65 years and those aged <65 years.11
No dose adjustment of capmatinib is required for patients with mild-to-moderate renal impairment. Data are not available on the use of capmatinib in patients with severe renal impairment.11
Warnings and Precautions
Interstitial lung disease (ILD)/pneumonitis, a potentially fatal condition, occurred in patients receiving capmatinib. Patients should be monitored for new or worsening pulmonary symptoms indicative of ILD/pneumonitis (eg, dyspnea, cough, and fever). Capmatinib should be permanently discontinued if the patient has ILD/pneumonitis and withheld if ILD/pneumonitis is suspected.11
Hepatotoxicity has occurred in patients receiving capmatinib. Liver function tests should be monitored prior to initiating capmatinib, every 2 weeks during the first 3 months of treatment, then once a month or as clinically appropriate, with more frequent testing in patients who develop increased transaminases or bilirubin. Capmatinib may require a dose reduction, or temporary or permanent discontinuation, depending on the severity of the adverse reaction.11
Photosensitivity may occur during treatment with capmatinib. Patients should be advised to limit direct ultraviolet exposure and take precautionary measures (eg, sunscreen or protective clothing).11
Pregnant women should be advised about the potential risk for fetal harm associated with the use of capmatinib. Women of reproductive potential should be advised to use effective contraception during capmatinib treatment and for 1 week after the last dose. Men with female partners of reproductive potential should be advised to use effective contraception during treatment and for 1 week after the final dose of capmatinib.11
Conclusion
Patients with metastatic NSCLC whose tumors harbor a MET exon 14 skipping mutation have limited treatment options. Capmatinib, a TKI, is the first therapy to receive FDA approval to treat this patient population. In the GEOMETRY mono-1 study, treatment with capmatinib showed an overall response rate of 68% in treatment-naïve patients and 41% in previously treated patients, and 47% and 32% of patients in these 2 groups, respectively, sustained their response for ≥12 months.11
The approval of capmatinib provides a therapeutic option that may improve outcomes for patients with metastatic NSCLC and the mutation leading to MET exon 14 skipping, as confirmed by an FDA-approved test.
References
- National Cancer Institute. Cancer stat facts: lung and bronchus cancer. https://seer.cancer.gov/statfacts/html/lungb.html. Accessed March 18, 2021.
- American Cancer Society. What is lung cancer? Updated October 1, 2019. www.cancer.org/cancer/lung-cancer/about/what-is.html. Accessed March 19, 2021.
- American Cancer Society. Lung cancer survival rates. Updated January 29, 2021. www.cancer.org/cancer/lung-cancer/detection-diagnosis-staging/survival-rates.html. Accessed March 19, 2021.
- Whisenant MS, Williams LA, Garcia Gonzalez A, et al. What do patients with non-small-cell lung cancer experience? Content domain for the MD Anderson Symptom Inventory for Lung Cancer. JCO Oncol Pract. 2020;16:e1151-e1160.
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines): Non-Small Cell Lung Cancer. Version 4.2021. March 3, 2021. www.nccn.org/professionals/physician_gls/pdf/nscl.pdf. Accessed March 19, 2021.
- Wolf J, Seto T, Han J-Y, et al; for the GEOMETRY mono-1 Investigators. Capmatinib in MET exon 14–mutated or MET-amplified non–small-cell lung cancer. N Engl J Med. 2020;383:944-957.
- Awad MM, Oxnard GR, Jackman DM, et al. MET exon 14 mutations in non–small-cell lung cancer are associated with advanced age and stage-dependent MET genomic amplification and c-Met overexpression. J Clin Oncol. 2016;34:721-730.
- König D, Prince SS, Rothschild SI. Targeted therapy in advanced and metastatic non-small cell lung cancer. An update on treatment of the most important actional oncogenic driver alterations. Cancers (Basel). 2021;13:804.9.
- Tong JH, Yeung SF, Chan AWH, et al. MET amplification and exon 14 splice site mutation define unique molecular subgroups of non-small cell lung carcinoma with poor prognosis. Clin Cancer Res. 2016;22:3048-3056.
- US Food and Drug Administration. FDA approves first targeted therapy to treat aggressive form of lung cancer. May 6, 2020. www.fda.gov/news-events/press-announcements/fda-approves-first-targeted-therapy-treat-aggressive-form-lung-cancer. Accessed March 10, 2021.
- Tabrecta (capmatinib) tablets, for oral use [prescribing information]. East Hanover, NJ: Novartis Pharmaceuticals Corporation; May 2020.