Ovarian cancer—including epithelial cancer, fallopian tube cancer, and primary peritoneal cancer—often affects women aged 55 to 64 years, with nearly 70% of all cases occurring in women aged ≥45 years.1 In 2020, an estimated 21,750 women will be newly diagnosed with ovarian cancer, and approximately 13,940 women will die from this disease in the United States.1
Ovarian epithelial cancer, fallopian tube cancer, and primary peritoneal cancer are characterized by the formation of malignant cells in the tissue covering the ovary or lining the fallopian tube or peritoneum.2 Overall, 58% of patients with ovarian cancer are diagnosed at an advanced stage, when the disease has already metastasized, affecting the rate of survival.1 The 5-year relative survival for women with advanced ovarian cancer is only 30.2%.1
Approximately 20% of ovarian cancers are caused by inherited genetic mutations, including BRCA1 or BRCA2 mutations.3 Poly (ADP-ribose) polymerase (PARP), an enzyme that helps to repair damaged DNA, plays a role in cellular processes, including replication and recombination.4 Tumors with BRCA1 or BRCA2 mutations are defined as having homologous recombination deficiency (HRD) repair status, which require PARP-mediated DNA repair to survive.4
The PARP inhibitors are targeted therapies that block malignant cells from repairing their damaged DNA, thereby causing cell death.5 The currently available PARP inhibitors niraparib, olaparib, and rucaparib are used in the treatment of certain previously treated patients with advanced ovarian cancer and a BRCA mutation, as well as for maintenance therapy after a partial or complete response to platinum-based chemotherapy, in patients with recurrent disease.3
The treatment options for advanced ovarian cancer include surgery, followed by platinum-based chemotherapy, alone or combined with taxanes or with other therapies.3 Postsurgical treatments may include systemic chemotherapy; intraperitoneal chemotherapy; chemotherapy and bevacizumab; or chemotherapy and a PARP inhibitor.3
Effective treatments are needed to improve outcomes for patients with advanced ovarian cancer who are at an increased risk for disease progression after first-line chemotherapy.6 Recently, one of the PARP inhibitors was granted an expanded indication as maintenance treatment for patients with advanced ovarian cancer, regardless of HRD status or BRCA mutation.7,8
Niraparib New Once-Daily Option for Advanced Ovarian Cancer
On April 29, 2020, the US Food and Drug Administration (FDA) granted the PARP inhibitor niraparib (Zejula; GlaxoSmithKline) a new indication for the maintenance treatment of all women with advanced epithelial ovarian, fallopian tube, or primary peritoneal cancer who have had a complete or partial response to first-line platinum-based chemotherapy, regardless of HRD status, such as the BRCA biomarker.7,8
The FDA granted niraparib priority review for this indication and was approved based on findings from the PRIMA study. Treatment with niraparib can be initiated without the need for an FDA-approved companion diagnostic.7
Commenting on the recent indication, Bradley Monk, MD, a PRIMA study investigator from the University of Arizona College of Medicine, Phoenix, said, “PRIMA was designed for patients with ovarian cancer who have a high unmet need. The positive data observed regardless of biomarker status in this study is extremely encouraging and suggests benefit beyond the BRCAm [mutation] population.”8
Niraparib was initially approved by the FDA in March 2017 for the treatment of recurrent ovarian cancer in women who have had a response to platinum-based chemotherapy.9 In October 2019, niraparib received a new indication for the treatment of women with recurrent ovarian cancer and HRD-positive status after ≥3 previous chemotherapy regimens.8,9
Mechanism of Action
Niraparib, an oral agent with antineoplastic activity, is an oral inhibitor of PARP-1 and PARP-2, enzymes that play a role in DNA repair. By binding to and inhibiting these enzymes, niraparib inhibits PARP-1 and PARP-2–mediated DNA repair and increases the formation of PARP-DNA complexes, resulting in DNA instability, apoptosis, and cell death. Increased niraparib-induced cytotoxicity has been observed in tumor cell lines with and without a BRCA msutation.10
Dosing and Administration
Niraparib is available as a 100-mg capsule for oral administration.10
The recommended dose of niraparib for the treatment of advanced ovarian cancer is 200 mg taken orally once daily for patients weighing <77 kg (<170 lb) or with a platelet count of <150,000/μL, and 300 mg taken orally once daily for patients weighing ≥77 kg (≥170 lb) and with a platelet count of ≥15,000/μL.10
Niraparib can be taken with or without food. Treatment should be continued until disease progression or until adverse reactions become unacceptable. If treatment toxicities occur, a treatment interruption, dose reduction, or dose discontinuation may be required.10
PRIMA Pivotal Clinical Trial
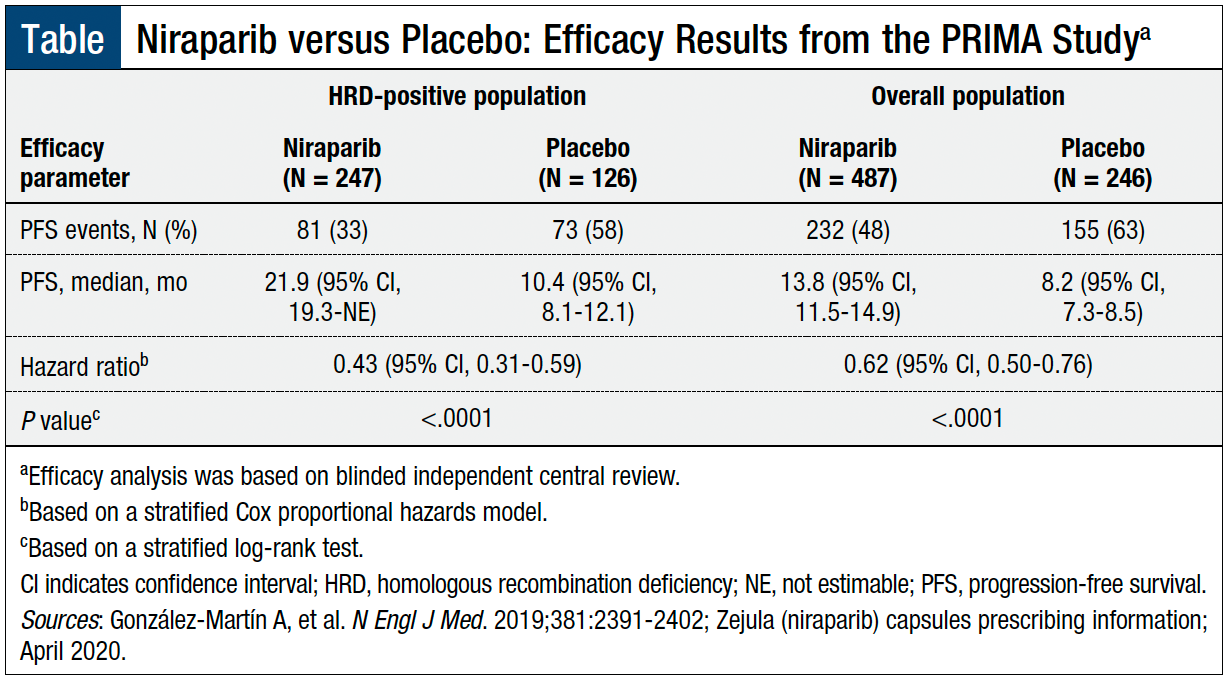
The efficacy and safety of niraparib were evaluated in the PRIMA study, a randomized, double-blind phase 3 clinical trial that included 733 patients (median age, 62 years; range, 32-85 years) who had a complete or partial response to first-line platinum-based chemotherapy.6,10 The patients were randomized in a 2:1 ratio to niraparib or to matched placebo after the completion of first-line platinum-based chemotherapy and surgery.10
The primary efficacy end point was progression-free survival (PFS), as determined by blinded independent central review, in patients with HRD-positive (as determined by the FDA-approved Myriad myChoice CDx assay) and in the overall population. HRD-positive status included having a BRCA mutation or a genomic instability score of ≥42. Overall survival was a secondary end point.10
Patients who received niraparib—in the HRD-positive population and in the overall population—had a statistically significant improvement in PFS compared with patients who received placebo (Table). The PFS was 11.5 months longer with niraparib therapy in patients with HRD-positive disease and 5.6 months longer in all patients, regardless of HRD status.10
At the 24-month interim analysis, the overall survival rate was 91% with niraparib versus 85% with placebo (hazard ratio [HR], 0.61; 95% confidence interval [CI], 0.27-1.39) in the HRD-positive population, and 84% versus 77%, respectively (HR, 0.70; 95% CI, 0.44-1.11), in the overall population.6
Adverse Reactions
Based on safety data from 3 pooled studies, the most common (≥10%) adverse reactions associated with niraparib were nausea (65%), thrombocytopenia (60%), anemia (56%), fatigue (55%), constipation (39%), musculoskeletal pain (36%), abdominal pain (35%), vomiting (33%), neutropenia (31%), decreased appetite (24%), leukopenia (24%), insomnia (23%), headache (23%), dyspnea (22%), rash (21%), diarrhea (18%), hypertension (17%), cough (16%), dizziness (14%), acute kidney injury (13%), urinary tract infection (12%), and hypomagnesemia (11%).10
In the PRIMA study, 32% of patients who received niraparib had serious adverse reactions; the rate of fatal reactions was 0.4%, including intestinal perforation and pleural effusion in 1 patient each. In all, 12% of patients permanently discontinued treatment because of adverse events.10
Niraparib has no contraindications.10
Use in Specific Populations
Data are not available on the effect of niraparib on human milk or on the breastfed infant or milk production. Women should not breastfeed during treatment and for 1 month after receiving the final dose of niraparib.10
In 2 clinical trials, no overall differences were seen in the safety and effectiveness of niraparib treatment between patients aged ≥65 years and those aged <65 years.10
For patients with mild-to-moderate renal impairment, no dose adjustment of niraparib is necessary. Data are not available on the safety of niraparib in patients with severe renal impairment or end-stage renal disease who are undergoing hemodialysis.10 For patients with mild hepatic impairment, no dose adjustment of niraparib is necessary. Data are not available on the safety of niraparib in patients with moderate-to-severe hepatic impairment.10
Warnings and Precautions
Cases of myelodysplastic syndrome (MDS) and acute myeloid leukemia (AML), including fatal cases, have been reported in patients who received niraparib therapy. Patients should be monitored for hematologic toxicity, and niraparib should be discontinued if MDS or AML is confirmed.10
Bone marrow suppression can occur in patients who receive niraparib therapy. Hematologic adverse reactions (ie, thrombocytopenia, anemia, and neutropenia) have been reported with niraparib. Patients should receive blood count tests weekly for the first month of niraparib therapy, monthly for the next 11 months, and periodically thereafter for clinically significant changes.10
Cardiovascular effects, including hypertension and hypertensive crisis, have been reported with niraparib. Monitor for blood pressure and heart rate at least weekly for the first 2 months of niraparib treatment, then monthly for the first year of treatment, and periodically thereafter during niraparib treatment. Antihypertensive medication and a niraparib dose adjustment may be necessary.10
Niraparib can cause fetal harm when administered to a pregnant woman. Women of reproductive potential should use effective contraception.10
Conclusion
The new indication for niraparib approved by the FDA provides a new first-line maintenance treatment option for all women, regardless of biomarker status, with advanced ovarian cancer who have had a complete or a partial response to first-line platinum-based chemotherapy. Niraparib is the first PARP inhibitor to be approved by the FDA for the first-line maintenance treatment of all women with ovarian cancer, regardless of any biomarker, including HRD-positive status or BRCA mutation. This latest indication for niraparib offers patients with advanced ovarian cancer a new once-daily treatment option.
References
- National Cancer Institute. SEER Program. Cancer stat facts: ovarian cancer. https://seer.cancer.gov/statfacts/html/ovary.html. Accessed May 22, 2020.
- National Cancer Institute. Ovarian epithelial, fallopian tube, and primary peritoneal cancer treatment (PDQ)–patient version. Updated May 20, 2020. www.cancer.gov/types/ovarian/patient/ovarian-epithelial-treatment-pdq. Accessed May 27, 2020.
- National Cancer Institute. Ovarian epithelial, fallopian tube, and primary peritoneal cancer treatment (PDQ)–health professional version. Updated December 18, 2019. www.cancer.gov/types/ovarian/hp/ovarian-epithelial-treatment-pdq#cit/section_1.19. Accessed May 26, 2020.
- Keung MYT, Wu Y, Vadgama JV. PARP inhibitors as a therapeutic agent for homologous recombination deficiency in breast cancers. J Clin Med. 2019;8:435.
- National Cancer Institute. PARP inhibitor. NCI Dictionary of Cancer Terms. www.cancer.gov/publications/dictionaries/cancer-terms/def/parp-inhibitor. Accessed May 27, 2020.
- González-Martín A, Pothuri B, Vergote I, et al; for the PRIMA/ENGOT-OV26/GOG-3012 investigators. Niraparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med. 2019;381:2391-2402.
- US Food and Drug Administration. FDA approves niraparib for first-line maintenance of advanced ovarian cancer. April 29, 2020. www.fda.gov/drugs/drug-approvals-and-databases/fda-approves-niraparib-first-line-maintenance-advanced-ovarian-cancer. Accessed May 18, 2020.
- GlaxoSmithKline. FDA approves Zejula (niraparib) as the only once-daily PARP inhibitor in first-line monotherapy maintenance treatment for women with platinum-responsive advanced ovarian cancer regardless of biomarker status. April 29, 2020. www.gsk.com/en-gb/media/press-releases/fda-approves-parp-inhibitor-in-first-line-monotherapy-maintenance-treatment-for-women-with-platinum-responsive-advanced-ovarian-cancer-regardless-of-biomarker-status/. Accessed May 27, 2020.
- Drugs.com. Zejula approval history. Updated April 30, 2020. www.drugs.com/history/zejula.html. Accessed May 22, 2020.
- Zejula (niraparib) capsules, for oral use [prescribing information]. Research Triangle Park, NC: GlaxoSmithKline; April 2020.
Used with permission from American Health & Drug Benefits. Copyright © 2020 Engage Healthcare Communications.