Immunotherapies have transformed the treatment of cancer, but patients who receive immune checkpoint inhibitors may experience a range of side effects, which are only recently becoming familiar to oncologists and patients. The novelty of immune checkpoint inhibitors has led the American Society of Clinical Oncology (ASCO), in collaboration with the National Comprehensive Cancer Network (NCCN) to jointly develop a new guideline to help clinicians recognize and manage these side effects.
The guideline was recently published in the Journal of Clinical Oncology,1 and the NCCN has issued its own version of the guideline as part of the NCCN Clinical Practice Guidelines (version 1.2018); the NCCN guidelines were presented at the recent NCCN annual conference (see “NCCN Issues First Guideline for Immunotherapy-Related Adverse Events”).
According to a joint press release from ASCO and the NCCN, “Patients who receive immune checkpoint inhibitors…may experience a unique set of side effects.”2
“These side effects,” the press release adds, “can involve multiple organs of the body, and although they are typically mild, sometimes severe, irreversible, or even life-threatening reactions can occur. Given that these therapies have entered the clinic fairly recently, few clinicians are experienced in recognizing and treating associated side effects.”2
The new recommendations apply only to side effects associated with immune checkpoint inhibitors and are not necessarily applicable to other types of immunotherapy.
Key Recommendations
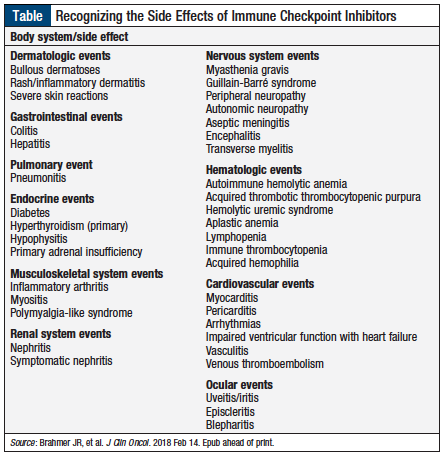
The recommendations address these immune-related side effects based on the various body systems (Table), along with recommendations for diagnostic testing.
The guidelines focus on recommendations to clinicians who are managing adults with cancer to help them manage these adverse events. The following are some of the key management recommendations, by the severity of each immune-related toxicity.1
Education
Oncologists and other providers must offer up-to-date education to patients and their caregivers regarding immunotherapies, including education about potential immune-related adverse events, before immune checkpoint inhibitors are administered, during treatment, and into survivorship. Providers are also advised to explain to patients and caregivers the mechanism of action of the specific treatment.
A presumption of guilt
Oncologists should view any new symptoms in a patient receiving immunotherapy with “a high level of suspicion,” because these may be related to the immune checkpoint inhibitor the patient is receiving.
Grade 1 toxicities
In general, patients should continue to use an immune checkpoint inhibitor but be closely monitored when grade 1 adverse events emerge. Exceptions include cardiac, neurologic, and hematologic adverse events.
Grade 2 toxicities
Treatment should be suspended when “most grade 2 toxicities” occur. Oncologists should consider resuming therapy when the patient’s symptoms and/or the laboratory values show a reduction to a grade 1 or lower adverse event. For such cases, patients should be given corticosteroids (ie, prednisone or its equivalent), with an initial dose of 0.5 to 1 mg/kg daily.
Grade 3 toxicities
Treatment with immune checkpoint inhibitors should be stopped for all grade 3 adverse events. Such patients should receive high-dose corticosteroids that “should be tapered over the course of at least 4 to 6 weeks.”1 Infliximab may be used for some adverse events if the patient’s symptoms do not improve within 48 to 72 hours of initiating high-dose corticosteroid therapy.
Rechallenge cautiously
Caution is advised when rechallenging with an immune checkpoint inhibitor after a patient’s symptoms or laboratory values return to grade ≤1 toxicity. Clinicians should be especially vigilant when attempting to rechallenge treatment in patients who had early-onset adverse events. Dose adjustments of immune checkpoint inhibitors is not recommended.
Grade 4 toxicities
Permanent discontinuation of immune checkpoint inhibitors is recommended when grade 4 toxicities occur, “with the exception of endocrinopathies that have been controlled by hormone replacement.”1
In addition, the NCCN version of the guidelines includes recommendations on infusion-related reactions, as well as principles for immunosuppression, patient education, immunotherapy rechallenge, and the routine monitoring of patients.3
Clinical Implications
With close monitoring, immune checkpoint inhibitors can typically be used even when patients have mild immune-related adverse events. Moderate-to-severe events “require early detection and proper management,”1 which is especially true for patients with a history of transplantation or preexisting autoimmune disease.
“Most of the side effects are reversible, but early recognition and proper treatment are critical,”2 advised John A. Thompson, MD, Co-Director, Seattle Cancer Care Alliance Melanoma Clinic, Fred Hutchinson Cancer Research Center, and co-chair of the expert panel that developed the guideline.
“These guidelines will help all providers who care for patients treated with immune checkpoint inhibitors, not just oncologists, but also emergency room and primary care doctors, assess and manage these side effects,”2 said Julie Brahmer, MD, chair of the expert panel.
“In most cases, irAEs [immune-related adverse events] can be managed with treatment interruption and/or supportive care and for some patients, will involve a multidisciplinary team,”1 according to the ASCO guidelines.
The goal is that, following these guidelines, a clinician will be equipped with “strategies and best practices to rapidly recognize, diagnose, coordinate with other medical subspecialties, and manage these sets of unique toxicities.”1
References
- Brahmer JR, Lacchetti C, Schneider BJ, et al; in collaboration with the National Comprehensive Cancer Network. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2018 Feb 14. Epub ahead of print.
- National Comprehensive Cancer Network; American Society of Clinical Oncology. Leading cancer organizations provide guidance on understanding and managing immunotherapy side effects. Press release. February 14, 2018. www.asco.org/about-asco/press-center/news-releases/leading-cancer-organizations-provide-guidance-understanding. Accessed February 21, 2018.
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Management of Immunotherapy-Related Toxicities (Immune Checkpoint Inhibitor-Related Toxicities). Version 1.2018. February 14, 2018. ww.nccn.org/professionals/physician_gls/default.aspx. Accessed February 14, 2018.